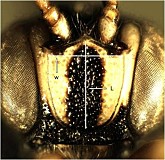
Metopius is readily characterized and separated from all other members of the Metopiinae by the distinctive shield-shaped carina that encompasses the face, forming a facial shield (Fig. 1, #3 and Figs 2-3). Additionally, the epipleurites (=lateral tergites) are always well-developed, there is a single tibial spur on the mesothoracic leg, and the first two metasomal tergites are fused.
The species of Metopius are highly color-variable and this variation has been used extensively to delimit species and subspecies. The following characters and character states encompass much of the remaining morphological variation in Metopius and these features were used to explore relationships among the recognized subgenera, as detailed in the sections above.
The interantennal process (#2 in Fig. 1) extends between the antennae, from the facial shield to the frons. It is present in all species of Metopius, exhibiting the following variation: Laterally compressed, with anterior portion extending forward onto the dorsal part of the shield as a ridge-like projection (Fig. 4); laterally compressed, at least dorsally, without anterior ridge (Fig. 5); anterior portion square to trapezoidal, concave, with raised marginal flanges (Fig. 6); anterior portion broadly triangular and flattened (Fig. 7); anterior portion broadly triangular, posterior portion concave, with raised marginal flanges (Fig. 8).
The frontal horn is a distinct projection physically separated from and posterior to the interantennal process. It is present in a few taxa, such as M. fuscipennis (Fig. 9) and M. metallicus, but absent in most or present but connected to the interantennal process by a distinctly elevated flange (as in M. birkmani).
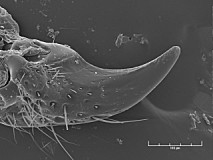
The lower or ventral tooth of the mandible may be either absent (Fig. 10) or present. When present, it may be represented by a small, inwardly-directed projection not separated by a groove from the dorsal tooth; it may be small and inwardly-projecting, but separated by a distinct groove or indentation from the dorsal tooth (Fig. 11); or it may be larger: only slightly smaller than the dorsal tooth and in the same plane as the dorsal tooth.
The occipital carina is present dorsally, but variously absent ventrally: either relatively complete and extending well below the level of the middle of the foramen magnum (Fig. 12) or relatively incomplete and extending ventrally at most to the middle of the foramen magnum.
Clypeal margin: the clypeus and face are not distinctly separated and the clypeal margin (Fig. 1, # 6) thus appears to be the ventral margin of the face. The margin may be carinate, with the carinate margin weakly protruding (Fig. 13); the entire clypeus may be sloping outwardly so that it overhangs the base of the labrum (Fig. 14); or the margin may be impressed and not distinctly protruding (Fig. 15).
The flagellum is normally cylindrical to slightly flattened in the middle, with the flagellomeres distinctly tapering distally (Fig. 16, middle and right). In some species, however, the flagellomeres are more strongly flattened in the distal third (Fig. 16, left).
The tarsal claws of the pro- and mesothoracic legs may be either simple (Fig. 17) or pectinate (Fig. 18). In nearly all species with pectinate claws, the pectination is confined to the basal half and is often weak (Fig. 19).
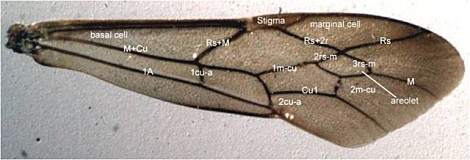
Cross-vein 2m-cu of the fore wing may have either 1 (Fig. 20) or 2 bullae. Unfortunately, this character is subject to some intraspecific variation. Removal of this character from our preliminary analyses resulted in fewer equally parsimonious trees.
The fore wing color pattern varies as follows: Uniformly colored, either hyaline or variously yellow (Fig. 20); bicolored, with anterior 1/3 of the wing dark brown from base to apex, remainder hyaline or nearly so; or bicolored but darkening distally (Fig. 21), with dark yellow to brown coloration limited to the marginal cell and apex of wing, cells basal to stigma never darkened.
The epicnemial carina has three character states, with the first two somewhat subjective: Carina absent dorsally, ending just above the sternaulus and widely separated from the anterior and dorsal margins of the mesopleuron (Fig. 22); carina gradually turns towards anterior margin of mesopleuron with no distinct turn, ending parallel at a distance equal to or greater than the width of an ocellus (Fig. 23); carina turns sharply towards anterior margin of mesopleuron above the sternaulus before turning again and ending at or closely parallel to anterior margin of mesopleuron (Fig. 24), leaving a width less than the diameter of an ocellus.
A tibial tooth is usually present as a narrow, distinctly pointed apical extension of the dorsal rim of the metathoracic leg, opposite the tibial spurs (Fig. 25), but is absent or nearly so and broadly rounded in some species.
The shape of the subalar prominence is considerably variable, and we attempted to capture this variability with the following character states: weakly convex; narrowly and more strongly convex; narrow and ridge-like.
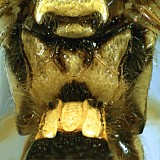
Fusion of the first and second metasomal tergites (T1 and T2) is characteristic of Metopius. Though fused, the suture is still visible as an impressed line, either continuously across the middle (that is, uninterrupted: Figs 26, 30) or the suture may be interrupted by a pair of longitudinal carinae from T1 (Fig. 27) that extend onto the base of T2, obliterating the suture line medially. In outgroup taxa Acerataspis, Hemimetopius, and Pseudometopius, there is no fusion of T1 and T2.
The male clasper is either evenly convex or depressed apical-ventrally, resulting in a lateral ridge.
Propodeal carinae are variously reduced or absent and the following characters states address this variation: greatly reduced, with no closed cells, anterior transverse carina (ATC) and posterior transverse carina (PTC) absent, short remnants of median longitudinal carinae anterior and lateral longitudinal carinae posteriorly usually present; pleural carina present (Fig. 28); moderately reduced: either ATC present laterally, but median longitudinal carinae mostly absent (Fig. 29); OR ATC largely absent but median basal area + areola distinct; nearly complete areolation, with closed cells anteriorly and laterally, but with most of PTC absent and basal area fused with areola, closed posteriorly by short median section of PTC, with median section of ATC absent (Figs 30, 31); completely areolate, ATC, lateromedian longitudinal carina, lateral longitudinal carina, and PTC present as strongly elevated carinae (condition in some outgroups, but not in any Metopius we have seen.