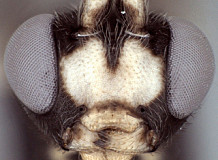
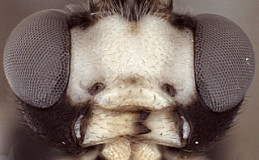
Frons without median horn or elevated carina. Clypeus small (Figs 3-6) narrow and short, with transverse, subapical bulge that is well developed and overlapping apical margin medially in some species (Fig. 3) but nearly absent in others; ventral margin otherwise sharp throughout, truncate (Fig. 3) or weakly concave (Fig. 4) medially, with sharp lateral margins distinctly angled dorsally; epistomal sulcus distinct in some species, indistinct in others. Malar space varying from about 0.4 to 0.9 times basal width of mandible. Mandible (Figs 3-6) curved, gradually narrowing from base to apex, ventral tooth varying from about equal in length to somewhat shorter than dorsal tooth and usually narrower than dorsal tooth; when mandibles closed, clypeus sometimes obscures part of mandible and mandible sometimes obscures part of clypeus. inner eye margins parallel to very weakly converging ventrally. Ocelli small, with maximum diameter of lateral ocellus distinctly shorter than distance between ocellus and eye. Female antenna equal to or longer than body. male antenna usually shorter than body; first flagellomere long and slender in one species examined, but shorter in others (as in Figs 1, 2). Hypostomal carina joining occipital carina well above base of mandible; occipital carina complete. Epomia absent. Dorsal end of epicnemial carina extending to anterior margin of mesopleuron in all specimens examined; mesopleuron finely to coarsely mat ventrally, nearly rugulose in some species. Notaulus sharply impressed on anterior declivity (though not quite reaching anterior margin in one of the species examined), distinctly impressed on disk at least to level of tegula. Pleural carina complete, well-developed; propodeum (Figs. 7, 8) with lateral longitudinal carina well-developed, complete or nearly so though usually weaker anteriorly; median longitudinal carinae forming a broad, rounded petiolar area then extending anteriorly as sharp, more narrowly spaced ridges; short, broad petiolar area usually with median longitudinal carina (Fig. 7) and separated by transverse carina from combined areola plus basal median area; posterior transverse carina present across middle, extending to lateral longitudinal carina; anterior transverse carina completely absent. Hind tibia with apical comb on posterior side absent or poorly developed; hind tibial spurs long, slender, spurs unequal in size, longest spur 0.5-0.8 times length of hind basitarsus; all tarsal claws usually pectinate, but extent and development of pectination varies among species. Fore wing (Fig. 9) with areolet absent; stigma not somewhat narrow, with Rs+2r arising from or a little basad midpoint. Hind wing (Fig 9) with first abscissa of CU1 longer to much longer than 1cu-a. T1 (Figs 10-12) relatively short and broad; gradually widening posteriorly; basal depression for dorsal tendon attachment deep; dorsal carinae always well-developed, converging from base towards rugose, transverse, subapical groove; dorsal-lateral carina sharp and distinct from spiracle to apex; glymma large, shallow relative to
Anoncus. S1 very short, 0.25-0.35 times length of T1. T2 thyridium distinct in all but one of species examined; laterotergites of T2 and T3 completely separated by creases. Shallow transverse grooves present subapically on T1, T2, and sometimes T3 (Fig. 12); T1 and T2 coarsely sculptured. Ovipositor fairly short (Fig. 13), straight, with well-developed subapical, dorsal notch; ovipositor sheath (Fig. 13) not parallel-sided. Apex of female metasoma as in Fig. 13.
This description is modified from that of Townes, 1970: 127 and based on 3-4 species in the Texas A&M University Collection.