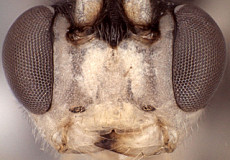
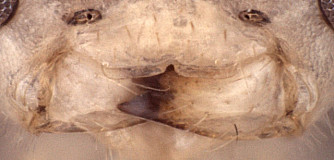
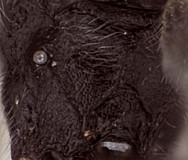
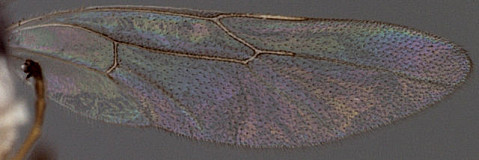
Zootrephes Foerster, 1869: 162. Type species: Bassus (Zootrephes) hilaris Woldstedt. Subsequently included by Woldstedt, 1880: 175. Monobasic. Synonymized under Synodites by Townes et al. (1965: 264).
Sychnoportus Foerster, 1869: 208. Type species: Sychnoportus rufopectus Ashmead, 1898 [= a junior subjective synonym of Synodites olympiae Ashmead, 1896]. Subsequently included by Ashmead (1898: 169). Monobasic. Synonymized under Polyterus by Townes (1945: 523).
Listrota Foerster, 1869: 209. Type species: Phobetes canadensis Harrington, 1894. First subsequent inclusion by Davis (1897: 289). Monobasic. Synonymized under Polyterus by Townes (1945: 523).
Polyterus Foerster, 1869: 209. Type species: Polyterus franconiaensis Davis, 1897. Subsequent designation by Viereck (1914: 121) from among two species first included by Davis (1897: 289). Synonymized under Synodites by Townes et al. (1965: 264).
Camponastes Foerster, 1869: 212. Type species: Camponastes basilicus Davis, 1897. First subsequent inclusion by Davis (1897: 292). Monobasic. Synonymized under Polyterus by Townes (1945: 524).
Sarcorychus Foerster, 1869: 212. Type species: Tryphon notatus Gravenhorst, 1829. Subsequent designation by Perkins (1962: 450). Synonymized under Synodites by Perkins (1962: 450).
Zootrephus Thomson, 1890: 1486. Unjustified emendation.
Synodytes Thomson (1894: 2002). Unjustified emendation.
The above synonymy follows Townes (1970: 131). Anaglymmus was included as a synonym of Synodites by Yu and Horstmann (1997). In Yu et al. (2012), the genus is listed as a synonym of Synodites, but the two originally included species are placed in Syndipnus. Townes (1970: 135) treats Anaglymmus as a synonym of Syndipnus. Lathrophagus has an even more convoluted history: it was first synonymized under Hypamblys by Perkins (1962), and this treatment is followed by Townes (1970: 136). Aubert (2000) lists it as a synonym of both Synodites and Hypamblys. Yu et al. (2012) includes the type species in Synodites, citing an inventory of a Dutch natural area by Zwakhals et al. (1996). Since the list of included species cited on this page is from Yu et al. (2012), it includes breviusculus, the current valid name for the type species of Lathrophagus.