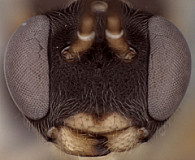
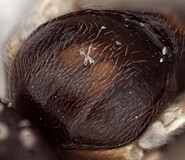
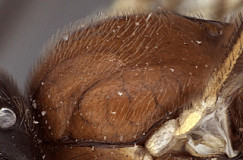
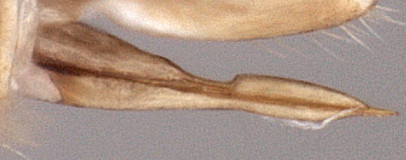
Gastroporus Foerster, 1869: 206 Type species: Hyperallus caliroae Viereck, 1911. Subsequent inclusion by Perkins (1962: 424). Synonymized by Perkins (1962: 424).
Hyperallus Foerster, 1868
The above description is considerably modified from Townes (1970), and based on the holotypes of Polyterus caliroae Rohwer and Hyperallus caliroae Viereck as well as four specimens in the Texas A&M University collection.
There are no specimens currently determined for this OTU, or those specimens determined for this OTU are not yet mappable.
This work would not have been possible without the groundwork provided by Ian Gauld’s study of the Australian and Costa Rican faunas, and we are particularly grateful for his assistance in many aspects of this study. We thank David Wahl of the American Entomological Institute, Gavin Broad of The Natural History Museum, London, Bob Kula of the USDA Systematic Entomology Laboratory, and Andy Bennett of the Canadian National Collection for extended loans of the material used for this study. We also thank David Wahl for useful feedback throughout our study. Matt Yoder provided considerable assistance with databasing issues, and our use of PURLs (http://purl.oclc.org) in this regard follows the example of their use in publications by Norm Johnson. Heather Cummins, Andrea Walker, Patricia Mullins, Caitlin Nessner, Mika Cameron, Karl Roeder, Danielle Restuccia, and Cheryl Hyde graciously assisted us with image processing, formatting, and literature retrieval. This study was supported by the National Science Foundation’s PEET program under Grant No. DEB 0328922 and associated REU supplement nos DEB 0616851, 0723663, 0822676, 0923134, and 1026618.
This material is based upon work at Texas A&M University supported by the National Science Foundation under Grant Number DEB 0328922 with REU supplements DEB 0616851, 0723663, 0822676, 0923134, and 1026618.. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.