Metopiinae Förster, 1869
Bremiella and Ischyrocnemis were removed to Ctenopelmatinae by Aubert (2000) without explanation. Gauld and Wahl (2006) presented excellent arguments for treating Scolomus Townes, 1950 in Townes and Townes, 1950 as a senior synonym of Apolophus and for placing this genus as a basal member of the Metopiinae. Yu et al. (2011) recorded the synonymy but failed to move Scolomus to Metopiinae, leaving it in the Ctenopelmatinae (Pionini). The placement of Lapton has not been questioned since Townes (1971) and thus Lapton remains in Metopiinae. As of 2013, therefore, Metopiinae includes 25 valid, extant genera, including Lapton and Scolomus but excluding Bremiella and Ischyrocnemis.
Extant genera are listed below, arranged as in Townes and Townes (1959) and discussed further in the Diagnosis and Relationships section. Two other genera, based on fossils, have also been described (Yu et al. 2011).
Group 1, Townes and Townes (1959):
First Subgroup:
Pseudometopius Davis, 1897
Acerataspis Uchida, 1934
Second Subgroup:
Chorinaeus Holmgren, 1858
Trieces Townes, 1946
Hemimetopius Benoit, 1955
Group 2, Townes and Townes (1959):
First Subgroup:
Triclistus Foerster, 1869
Cubus Townes and Townes, 1959
Colpotrochia Holmgren, 1856
Second Subgroup of Group 2:
Spudaeus Gistel, 1848
Third Subgroup of Group 2:
Periope Haliday, 1839
Bothromus Townes and Townes, 1959
Drepanoctonus Pfankuch, 1911
Leurus Townes, 1946
Seticornuta Morley, 1913
Carria Schmiedeknecht, 1924
Macromalon Townes and Townes, 1959
Hypsicera Latreille, 1829
Stethoncus Townes and Townes, 1959
Synosis Townes and Townes, 1959
Exochus Gravenhorst, 1829
Isolated placement in Metopiinae:
Metopius Panzer, 1806
Genera of uncertain placement, vide Townes (1971):
Lapton Nees von Esenbeck, 1816
Ischyrocnemis Holmgren, 1856 (now in Ctenopelmatinae, Pionini, vide Aubert 2000)
Bremiella Dalla Torre, 1902 (now in Ctenopelmatinae, Perilissini, vide Aubert 2000)
Apolophus Townes, 1971
Described subsequent to work of Townes, 1971:
Sciron Fitton, 1984
Forrestopius Gauld and Sithole, 2002
The Metopiinae are often characterized as stout species with bulging faces and shortened fore tarsi (Townes and Townes 1959, Broad and Shaw 2005). More specifically they have been defined by the absence of a distinct epistomal groove, presence of an ovoid scape and short ovipositor, placement of the spiracle of the petiole near its midlength, and by fore and mid legs with trochantellus usually fused to femur (Townes and Townes 1959). Townes and Townes (1959) indicated that the most distinctive feature was the protrusion of the upper margin of the face between or above the bases of the antennae, though Gauld and Sithole (2002) emphasized the absence of an epistomal sulcus between the lower face and clypeus as the basis for metopiine monophyly. Aside from these observations, the monophyly of the subfamily, though never doubted, has not been examined in detail from a morphological perspective. Further, Townes (1971) subsequently included two genera, Apolophus (now a junior synonym of Scolomus) and Ischyrocnemis, whose species lack the distinctive protrusion of the face between or above the antennal bases. At least some of the Scolomus that we have examined also have a weak epistomal sulcus. Based on the absence of several of the distinctive morphological features of other metopiines, Gauld and Sithole (2002), Broad and Shaw (2005), and Gauld and Wahl (2006) suggested that Scolomus is likely to be one of the more basal members of the Metopiinae.
Retention of Scolomus in Metopiinae leaves few, if any, morphological synapomorphies for the subfamily. In Scolomus, the face is neither bulging nor protruding above or between the antennal bases, there is a weak epistomal sulcus, and the trochantellus is distinct on the fore and mid legs. Recent authors, in an attempt to resolve the issue of where to place Scolomus, have emphasized the importance of short tarsomeres of the fore and middle leg of the type species of Apolophus (Broad and Shaw 2005) and in particular the transverse tarsomere 4 of the fore leg (Gauld and Sithole 2002, Gauld and Wahl 2006) for retaining Scolomus in Metopiinae. Our brief examination of representative genera, however, reveals that a transverse tarsomere 4 is not found in all metopiines (some Drepanoctonus, for example), nor in all species of Scolomus (notably some of the Chilean species). If size of the fore leg tarsomeres is potentially the most important morphological synapomorph for defining Metopiinae, a more rigorous assessment of this feature across the Metopiinae, in comparison with other putatively basal Ophioniformes, is needed.
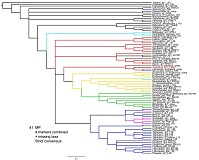
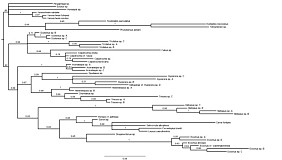
Quicke et al. (2009) presented a phylogeny of the Ichneumonidae in which they included 20 of the valid metopiine genera plus Bremiella and Ischyrocnemis (Bremiella and Ischyrocnemis were both included in Metopiinae in their study). Results were based on parsimony analyses of a dataset consisting of 28S rDNA sequences plus a heavily weighted morphological matrix. Scolomus, Bremiella, and Lapton failed to cluster with the remaining Metopiinae, but the remainder did form a monophyletic group. However, metopiines were one of several non-ctenopelmatine groups embedded in the Ctenopelmatinae, rendering the latter paraphyletic. Our preliminary results assessing relationships among Ctenopelmatinae, based on partial sequence data from 28S, CAD, and Pol2, also resulted in the few metopiine exemplars embedded deeply within Ctenopelmatinae (Fig. 1, parsimony analysis and Fig. 2. Bayesian analysis, both conducted by J. Dubois).
Relationships among Genera.
Apart from Townes and Townes (1959) and Quicke et al. (2009), there has been almost no discussion of the relationships among metopiine genera. Townes and Townes (1959) indicated that there were several “natural groups” of genera, some of these well-delineated and others poorly so. These “natural groups” are listed above in the Taxonomic History/Nomenclature section, arranged as found in Townes and Townes (1959). (Townes and Townes referred to them simply as groups and subgroups, we have added numbers to facilitate discussion.) Townes and Townes (1959) placed Pseudometopius, Acerataspis, Chorinaeus, Trieces, and Hemimetopius (which we refer to above as Group 1) together and provided a diagnosis in the form of a series of explicit morphological features, one of which (reduced epipleurites) is unique. They further separated Pseudometopius and Acerataspis from the other three genera, with each of these two subgroups clearly defined in the third couplet of their key to genera. Metopius, uniquely defined by the facial shield, was characterized as isolated within the Metopiinae by Townes and Townes (1959). The remaining Metopiinae were treated as a “loose grouping of genera” by Townes and Townes (1959) but one small subgroup, consisting of Triclistus, Cubus, and Colpotrochia, was explicitly defined as a natural group, with Spudaeus treated as “somewhat similar” and placed on its own in a second subgroup. The list of genera in the third subgroup is the arrangement in Townes and Townes (1959), reflecting a series of “individual and progressive specialization, leading ultimately to Exochus as the most specialized genus of the subfamily.”
The relationships suggested by Townes and Townes (1959) are mirrored in the key of Townes (1971), in which Groups 1 and 2 are defined by unambiguous characters in the first half of the key. In published keys to metopiine genera (Townes and Townes 1959, Townes 1971, Fitton 1984, Gauld and Sithole 2002), Metopius is always removed in the first couplet because of the distinctive facial shield. This limits the utility of these keys for acquisition of character states that might be useful for exploring relationships of Metopius to other Metopiinae. As noted by Townes and Townes (1959), Metopius shares some characteristics of group 1, but lacks the unique feature of reduced epipleurites.
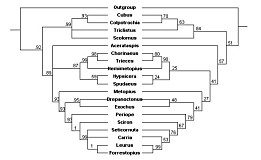
The monophyletic cluster of 18 genera resolved in the analyses of Quicke et al. (2009) exhibited the following set of relationships: ( Colpotrochia + Cubus + Triclistus ) + (( Exochus + Hypsicera ) + (( Metopius + Acerataspis ) + (( Hemimetopius + Chorinaeus + Trieces ) + ( Spudaeus + Drepanoctonus + Periope + Sciron + Carria + Seticornuta + Leurus + Forrestopius )))). The natural classification proposed by Townes and Townes (1959) was largely corroborated by Quicke et al. (2009). Notable exceptions include the sister-group relationship between Metopius and Acerataspis in Quicke et al. (2009) and the more isolated position of Exochus + Hypsicera relative to the other members of the third subgroup Group 2 of Townes and Townes (1959). The flange-like carinae bordering the scutellum in Metopius could be used to support the association of Metopius with Acerataspis. This characteristic is relatively widespread among the members of Group 1 but apparently absent in group 2. Bothromus, Macromalon, Stethoncus, and Synosis, all members of the third subgroup of Townes and Townes’s group 2, were not present in the analyses of Quicke et al. (2009).
In their analyses of 630 named genera of Ichneumonidae, Quicke et al. (2009) combined molecular and morphological datasets to portray relationships. All Metopiinae, however, were coded uniformly for morphological data (with the exception of Bremiella, Ischyrocnemis, Lapton, and Scolomus). Unfortunately, because the species are so poorly known, at least one-third of the morphological dataset was coded as missing for each of these four genera Quicke et al. (2009). It is therefore not surprising, given the heavy weighting of the morphological dataset, that these four genera did not cluster with the remaining Metopiinae. Thus, in our reassessment, we focused solely on the molecular data (28S, D2-D3).
Quicke et al. (2009), aligned the 28S rDNA data using a variety of computational methods. Alignment based on the purported secondary structure of the rRNA better encapsulates the phylogenetic signal for phylogenetic analysis (Dixon and Hillis 1993, Kjer 1995); and Gillespie et al. (2005) presented a secondary structure model for Ichneumonidae 28S rRNA. Telford et al. (2005) subsequently demonstrated the utility of a doublet model in allowing pairing and non-pairing regions to be treated separately in phylogenetic analyses. These approaches allow more information regarding the phylogenetic signal to be extracted from the data. While their use for very large datasets might be challenging, they are relatively easy to implement for smaller ones. We therefore used the GenBank accession numbers in Quicke et al. (2009) to acquire their 28S D2 and D3 sequences, which we then used to perform secondary structural alignments (following protocols outlined by Gillespie et al. 2005 and Yoder and Gillespie 2004a, b) for further exploring the metopiine portion of the 28S dataset. We added 5 sequences that we had generated from Scolomus, Metopius, Hemimetopius, and two species of Exochus, and deleted the Quicke et al. sequence of Carria sp. because of the large amount of missing data. Our 5 additional sequences were submitted to GenBank and issued the following accession numbers: D2 sequences (GQ466083, GQ466084, GQ466085, GQ466086, GQ466087); and D3-D5 sequences (GQ466088, GQ466089, GQ466090, GQ466091, GQ466092). Voucher specimens from which the legs were removed are deposited in the Texas A&M University Insect Collection as voucher #661.
For our reanalyses (Figs. 3 and 4), maximum likelihood and Bayesian analyses were performed to reconstruct the phylogeny. For the maximum likelihood analysis, RAxML (Stamatakis 2006) was used, with the GTR+I+G model for the final ML search and GTR+CAT for 32000 rapid bootstrap replicates. MrBayes (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003) was used, with a doublet model and unlinked GTR+I+G models for the paired and unpaired nucleotides. Four runs of four chains each were performed and run for ten million generations each, sampling every thousand generations. A burn-in of 20 thousand was used after stationarity was verified using Tracer (Rambaut & Drummond 2007: Tracer v1.4.1, Available from http://beast.bio.ed.ac.uk/Tracer).
Our results are shown in Figs. 3 and 4. The Hypsicera + Spudaeus clade shown in Fig. 3 was recovered unambiguously in the Bayesian analysis, using the doublet model (results on left side), and in the maximum likelihood analyses (results on right side) that excluded Scolomus, Lapton, Bremiella, and Ischyrocnemis, but was not recovered in the parsimony analyses (not shown). Parsimony analyses (one with gaps treated as uninformative and one with gaps treated as informative) otherwise yielded the same pattern of relationships shown in Fig. 3: most clades were fully supported, but the relative positions of Acerataspis, Hypsicera, Spudaeus, Metopius, Exochus, and Drepanoctonus were ambiguous. Figure 4 shows all taxa from the Bayesian analysis using the doublet model, excluding Scolomus, Lapton, Bremiella, and Ischyrocnemis and provides names for the individual taxa including the outgroups (two banchines, a tryphonine, and several ctenopelmatines).
Our analyses, contrary to that of Quicke et al. (2009), leave the position of Metopius unresolved relative to Groups 1 and 2 of Townes and Townes (1959), thus supporting the ambiguous placement by Townes and Townes (1959). Otherwise, our analyses recover the larger groups and subgroups proposed by Townes and Townes (1959). For example, Chorinaeus + Hemimetopius + Trieces is very strongly supported as is Cubus + Colpotrochia + Triclistis. No molecular data are available for Pseudometopius, which helps to explain the more ambiguous position of Acerataspis in the molecular analyses. In our analyses, Scolomus falls well within the Metopiinae, usually associated with the Cubus + Colpotrochia + Triclistis clade even though Scolomus lacks the distinctive interantennal flange, noted by Townes and Townes (1959), that is shared by the species in these other three genera.
Another strongly supported set of relationships in our analyses was a clade containing Carria, Leurus, Seticornuta, Sciron, and Forrestopius. Although the latter two genera were described subsequent to Townes (1971), an association comprised of Carria, Leurus, and Seticornuta was suggested by Townes (1971) when he stated that there are some species whose morphology tends to blur the limits of these three genera. These five taxa form part of a larger clade, recovered in both our analyses and those of Quicke et al. (2009), that coincides largely with the third subgroup of Group 2 that was recognized by Townes and Townes (1959).
There are no specimens currently determined for this OTU, or those specimens determined for this OTU are not yet mappable.
A few additional details of the unpublished analyses shown here are available from Wharton. Page last updated April, 2014.
This material is based upon work supported by the National Science Foundation under Grant Number DEB 0328922 and associated REU supplement # 0616851 as well as NSF/REU grant # 0755264 to the Texas A&M Department of Entomology.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.