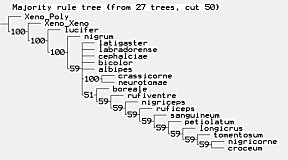
Representatives of all of the Nearctic species groups delineated by
Barron (1981) were obtained for the phylogenetic analyses whose results are presented in the diagnosis and relationships section below. Twenty-one species (19
Ctenopelma and two
Xenoschesis as outgroups) and 21 characters were used in these analyses. In addition to Nearctic species, some of the more distinctive species from the Palaearctic Region were used, including
Ctenopelma nigrum Holmgren, the type species of
Ctenopelma, and two members from each of the two major species groups proposed by
Kasparyan (2004). Taxa were chosen on the basis of availability, and to represent the range of variation within and among species groups indicated by
Barron (1981). Outgroup taxa were selected from the genus
Xenoschesis, which shares with
Ctenopelma relatively unmodified apical metasomal segments. Two North American species of
Xenoschesis were used, one from each of the two currently valid (Townes 1970) subgenera. The included species are listed at the end of this section.
Character data were analyzed under parsimony with WinClada (Nixon 2002) using branch and bound in TNT. All multistate characters were treated as nonadditive.
Characters and character states.—-Barron (1981) recognized six species groups of Nearctic Ctenopelma and provided a table with 17 characters for differentiating these groups. Unfortunately, some of the characters in the Barron (1981) revision are difficult to code unambiguously while others are not clearly defined. Barron (1981) used several character states associated with the petiole but we were unsuccessful in coding most of these as discrete character states. Figure numbers associated with various character states refer to figures in the description section below.
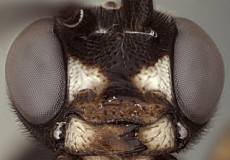
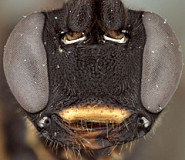
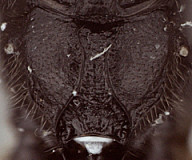
1. Female clypeus, dorsal and ventral divisions: 0) dorsal part shorter than ventral part (Fig. 3), usually distinctly so (Fig. 5) (separated by weak to strong, rounded transverse ridge); 1) dorsal and ventral parts roughly equal in size, or ventral part (depending on angle of view) slightly shorter, separated by rounded, transverse ridge (Figs 4, 6); 2) ventral part greatly reduced compared to dorsal part, and reflected inwardly medially (Fig. 7, Xenoschesis).
This character was intraspecifically variable to some extent, and hence difficult to code unambiguously for several species (particularly C. ruficeps Barron,
C. labradorense (Davis), C. bicolor Barron and C. cephalciae Barron). Sexual dimorphism was also apparent.
2. Female clypeus, shape of ventral margin: 0) deeply emarginate (Figs 3, 5); 1) shallowly emarginate but retaining median notch (Fig. 4); 2) more or less truncate, but retaining median notch; 3) truncate or broadly and weakly concave, without median notch (Fig. 7, bicolor).
The deeply emarginate clypeus is more rounded ventrolaterally and extends to the eye margin or nearly so whereas the shallowly emarginate clypeus is slightly more angular ventrolaterally and is more noticeably separate from the eye margin. The emargination of the clypeus was difficult to code because emargination is variable and can be interpreted differently depending on the angle of view.
3. Hypostomal carina: 0) meeting occipital carina distinctly above base of mandible; 1) separated from occipital carina ventrally; 2) meeting at base of mandible, or just slightly above.
In some species coded as 2, the carinae meet in a weakly elevated boss at the base of the mandible. These include C. crassicorne Walley and several members of the C. labradorense species group as defined by Barron (1981), but not C. nigricorne.
4. Female flagellomeres: 0) more than 30 flagellomeres, flagellomere 15 longer than wide; 1) less than 30 flagellomeres, flagellomere 15 at most as long as wide.
5. Color of female antenna: 0) pale with lighter apex; 1) all black, or at least dark dorsally; 2) similar to state 0 but dark at base, pale at apex; 3) similar to state 2 but darker at tip (i.e. mostly ferruginous, but dark basally and apically); 4) pale at base, dark at tip.
Most species coded as 1 have the flagellum entirely dark, but in C. petiolatum Barron and C. latigaster Barron, the flagellum is dark dorsally but paler ventrally. We coded these two species separately in a preliminary analysis, but the resulting consensus tree was the same so we have left them coded as 1.
6. Tarsal claw pectination in males: 0) all claws densely pectinate (Fig. 9); 1) all claws simple, not pectinate (Fig. 10); 2) some claws weakly, sparsely pectinate (Fig. 9), others densely pectinate.
Pectination of the tarsal claws is sexually dimorphic in many of the species and notably so in members of the C. labradorense species group delineated by Barron (1981). Pectination pattern was challenging to code for some species where pectines were larger on some tarsal claws than on others.
7. Tarsal claw pectination in females: 0) complete and dense on all three legs; 1) sparse, not extending to apex, but at least one tooth present distad base; 2) weak cluster only at extreme base.
Pectination was generally the same on all claws, but often difficult to see on sparsely pectinate claws because of unfavorable angle of view or broken pectines.
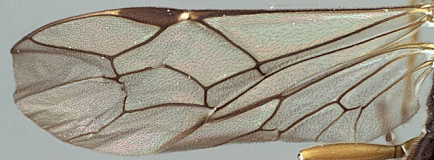
8. Number of bullae in fore wing cross-vein 2m-cu: 0) 2; 1) 1.
In C. croceum, the males examined had a single bulla but females had the bulla interrupted by a very short, sclerotized, often spur-like section, resulting in two bullae.
9. Fore wing areolet: 0) consistently absent (cross-vein 3rs-m absent) (Fig. 12); 1) present (Fig. 11), only rarely absent.
Of the species available, the areolet was absent only in C. nigrum. Few specimens of this species were available, and the stability of this character state in C. nigrum thus needs to be verified. The character does not contribute to assessment of relationships among species and is included here only because C. nigrum is the type species of Ctenopelma.
10. Lateral longitudinal carina of propodeum: 0) absent (Fig. 14); 1) present (Fig. 13).
For the species coded as absent, there is sometimes a short spur extending anteriorly from the top of the posterolateral area; a weak ridge may also be present anteriorly. Most species coded as present have a strong carina extending anteriorly to the transverse groove at the base of the propodeum. The carina is weaker medially in C. nigrum and C. lucifer.
11. Median portion of posterior transverse carina of propodeum: 0) absent (Figs 14, 15); 1) present between lateromedian longitudinal carinae, separating areola from petiolar area (Fig. 13).
12. Dorsal carinae of petiole: 0) absent; 1) weak; 2) well developed (Figs 19, 21).
State 1 is represented by species in which the dorsal carinae are low and somewhat rounded, not elevated and sharp. Character states apply specifically to middle portion of T1; not basal or apical portions, where the carinae are often absent regardless of how well developed they are medially.
13. Mid-dorsal impressions on petiole: 0) absent; 1) present at least medially (between spiracles); 2) absent medially, present subapically.
14. Glymma: 0) present, large, deep (Fig. 1); 1) absent (Fig. 2).
15. Tergite 2 sculpture: 0) uniformly deeply, densely punctate (Fig. 22); 1) strongly and extensively matt or granular matt; 2) polished, smooth to faintly and very sparsely punctate (Fig. 23).
In C. bicolor and C. cephalciae, coded as variable, the sculpture is both densely punctate and granular matt; in C. sanguineum, also coded as variable, the punctation is weaker than in state 0 but stronger and more extensive than in state 2.
16. Tergite 2 setal pattern: 0) uniformly, densely setose (Fig. 22); 1) decidedly sparsely setose.
17. Tergite 2 carina between spiracle and anterior margin.: 0) well developed, sharp and elevated throughout; 1) sharp basally, weak, rounded over apical half.
The carina is weak to virtually absent throughout in the specimen of C. crassicorne that we examined. It was coded as 1.
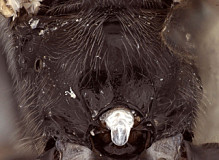
18. Ovipositor sheath shape: 0) moderately short, broad, nearly parallel-sided for most of length, rounded and weakly narrowed apically (Fig. 30); 1) similar to state 0 but strong subapical excavation dorsally, thus pointed apically (Figs 25, 26); 2) long, tapered apically, rounded at tip (Fig2 27, 31); 3) similar to state 1, with apex pointed but subapical excavation weak to absent (Fig. 24); 4) short, expanded distally (Fig. 32).
The sheath is somewhat rounded apically with the dorsal margin sinuate in C. sanguineum (Provancher), which differs from all other species examined. The rounded apex is most similar to the condition found in species such as C. croceum, however, and the species was therefore coded as 0. The shape of the sheath in Xenoschesis (Polycinetis) esplendens (Holmgren) was too dissimilar to that of other species and was therefore coded separately. Slight variation among species and preservation artifacts made coding of this character challenging for states 1 and 3. For this reason, separate analyses were performed with all of these species coded identically.
19. Ovipositor sheath length: 0) 2-3 x width; 1) > 4 x width (Figs 27, 31).
Although the sheath in C. lucifer (Gravenhorst) is longer than the sheath in Xenoschesis (Xenoschesis) cinctiventris (Ashmead), both have distinctly longer, more tapered sheaths than the other species and have therefore been coded identically.
20. Ovipositor sheath setae: 0) densely setose, setae separated by less than half their length; 1) sparsely setose, with long setae separated by at least half their length.
In Xenoschesis (Polycinetis) resplendens (Holmgren), setal density was somewhat intermediate between the two character states and was therefore coded for both.
21. Ovipositor: 0) with subapical notch (Figs 27, 30, 33); 1) without subapical notch (Fig. 24) .
Ctenopelma species included in the analysis, arranged by the species groups delimited by Barron, followed by 4 Palearctic species not readily assignable to these species groups:
nigricorne species group: Ctenopelma croceum Walley, C. nigricorne (Provancher), C. tomentosum (Desvignes), C. albidum Barron; sanguineum species group: C. sanguineum (Provancher), C. ruficeps Barron, C. nigriceps Barron; petiolatum species group: C. petiolatum Barron and C. longicrus Barron; crassicorne species group: C. crassicorne Walley and C. neurotomae Barron; albipes species group: C. albipes Barron and C. latigaster Barron: labradorense species group: C. bicolor Barron, C. cephalciae Barron, C. labradorense (Davis); C. boreale Holmgren, C. rufiventre (Gravenhorst), C. nigrum Holmgren, C. lucifer (Gravenhorst).