Nephopheltes Cushman, 1924: 16. Type species: Nephopheltes japonicus Cushman, 1924. Original designation. Synonymized by Townes (1945).
Opheltes Holmgren, 1859
Opheltes glaucopterus (Linnaeus, 1758)
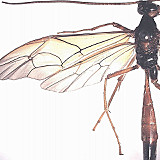
Opheltes japonicus (Cushman, 1924)
The latest treatments (such as Yu et al. 2005) list a number of synonyms as well as subspecies, somewhat complicating the ability to differentiate the taxa.
There are no specimens currently determined for this OTU, or those specimens determined for this OTU are not yet mappable.
This material is based upon work supported by the National Science Foundation under Grant Number DEB 0328922 with REU supplements DEB 0723663 and number 1026618.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.