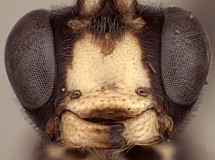
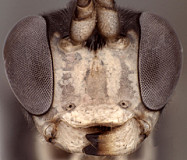
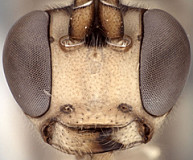
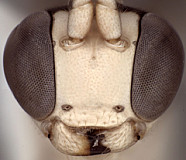
Frons without median horn or elevated carina. Clypeus (Figs 7-11) short and wide, strongly bulging subapically, with rounded transverse ridge; ventral margin sharp throughout, deeply impressed medially and occasionally difficult to see medially (Fig. 7) because of partially overlapping transverse ridge; ventral margin truncate to weakly concave medially, with lateral margins distinctly angled dorsally; epistomal sulcus sharply setting off face from bulging (in profile) clypeus. Malar space shorter than half basal width of mandible, very short in some individuals. Mandible (Figs 7-11) moderately long, curved, gradually narrowing from base to dark edge of apical teeth, then parallel-sided or slightly broadened, ventral tooth varying from slightly (Figs 7-9) to distinctly (Figs 10, 11) longer than dorsal tooth, with the shorter variant possibly due to wear. Inner eye margins parallel (Fig. 10) to weakly converging (Fig. 11). Female ocelli small, with maximum diameter of lateral ocellus distinctly shorter than distance between ocellus and eye, ocellus slightly larger in males examined. Female and male antennae longer than body; first flagellomere long and slender, nearly twice longer than second (Figs 1, 2). Hypostomal carina joining occipital carina above base of mandible; occipital carina complete. Epomia absent. Dorsal end of epicnemial carina usually extending to anterior margin of mesopleuron (Fig. 12) or nearly so, less commonly distinctly separated from anterior margin; mesopleuron ventrally mat, finely punctate. Notaulus absent (Fig. 14) to weakly impressed on anterior declivity (Fig. 13), weak to absent on disk. Pleural carina (Figs 15, 16) usually distinct dorsoanteriorly, weak to absent ventroposteriorly; propodeal carinae reduced to short spurs off posterior margin (Figs 15-17). Legs with apical comb on posterior side of hind tibia short but well developed, dense; hind tibial spurs long, slender (Figs 3, 4), longest spur often longer than half length of hind basitarsus; all tarsal claws apparently simple, not pectinate. Fore wing areolet present; stigma as in Fig. 6, not exceptionally broad, with Rs+2r arising from or near midpoint. Hind wing with first abscissa of CU1 usually slightly longer than 1cu-a, sometimes equal in length and rarely very slightly shorter. T1 (Figs 17-20) gradually widening posteriorly; dorsal carinae usually absent or confined to margins of deep basal depression of dorsal tendon attachment; dorsal-lateral carina sharp and distinct from spiracle to posterior margin of T1, often absent or poorly indicated anteriorly; glymma deep, broad basally, narrowing posteriorly. S1 extending to level of spiracle in most species, nearly so in others. T2 thyridium present; laterotergites of T2 and T3 completely separated by creases. Ovipositor (Figs 1, 2, 21, 22) short, more or less straight, with deep, broad subapical, dorsal notch; ovipositor sheath shorter than hind tibial spur. Apex of female metasoma as in Figs 21, 22.
This description is considerably modified from Townes (1970) and based largely on five Nearctic species in the Texas A&M University Collection.
According to Aubert (2000), some Palaearctic species have poorly developed glymmae similar to that of the type species of Apholium and he therefore treated Apholium as a synonym of Barytarbes_. The five North American species that we have examined all have a well-developed, deep glymma as in Fig. 19. See the _Apholium page for images of the type species of this nominal taxon.
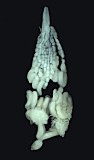
1.
Barytarbes provancheri habitus...
Barytarbes provancheri habitus
↰
↴
2.
Barytarbes honestus habi...
Barytarbes honestus habitus female
↰
↴
3.
Barytarbes ...
Barytarbes habitus female
↰
↴
4.
Barytarbes habitus femal...
Barytarbes habitus female
↰
↴
5.
Barytarbes habi...
Barytarbes habitus male
↰
↴
6.
Barytarbes h...
Barytarbes habitus male
↰
↴
7.
Barytarbes face and mandibles...
Barytarbes face and mandibles
↰
↴
8.
Barytarbes face and mandibles...
Barytarbes face and mandibles
↰
↴
9.
Barytarbes face and mand...
Barytarbes face and mandibles
↰
↴
10.
Barytarbes face and mandibles...
Barytarbes face and mandibles
↰
↴
11.
Barytarbes face and mandible...
Barytarbes face and mandibles
↰
↴
12.Barytarbes mesosoma and T1
16.
Barytarbes propodeum...
Barytarbes propodeum
↰
↴
17.
Barytarbes pr...
Barytarbes propodeum and T1
↰
↴
18.
Barytar...
Barytarbes T1 and T2
↰
↴
19.
Barytarbes metasoma female...
Barytarbes metasoma female
↰
↴
20.
Barytarbes metasoma male...
Barytarbes metasoma male
↰
↴
21.
Barytarbes female apex o...
Barytarbes female apex of metasoma
↰
↴
22.
Barytarbes female apex o...
Barytarbes female apex of metasoma
↰
↴
23.
Barytarbes ...
Barytarbes reproductive tract, female
↰
↴