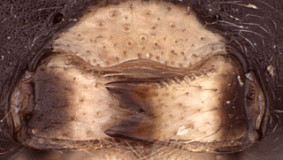
Clypeus (Figs 2, 3) narrow, with surface finely punctate; ventral margin sharp, but not distinctly impressed, more or less truncate to weakly concave medially, sharply angled towards face laterally; epistomal sulcus narrow, very shallow and poorly indicated in females examined, distinct in the one male examined; clypeus in profile flat in females (Fig. 1) to weakly bulging in males. Inner eye margins parallel. Malar space (Fig. 2) well-developed, varying from 1.0 to slightly less than 0.5 times basal width of mandible; malar sulcus absent. Mandible (Fig. 3) tapering gradually from base to apex; dorsal tooth broader and about equal in length to ventral tooth; ventral margin distinctly carinate. Maxillary palp shorter than height of head; antenna (Fig. 4) equal to or slightly shorter than body, first flagellomere short relative to species in genera such as
Mesoleptidea and
Hadrodactylus. Ocelli small, diameter of lateral ocellus less than distance from lateral ocellus to eye; the one male examined has slightly larger ocelli than the females examined. Hypostomal carina meeting occipital carina distinctly above base of mandible; occipital carina complete dorsally. Epomia present, though sometimes weak. Epicnemial carina not or only rarely reaching anterior margin of mesopleuron. Notaulus present usually as a deep, distinct impression on anterior declivity (the depression sometimes weakly sculptured), becoming distinctly weaker and shallow on disk, usually extending posteriorad level of tegula. Groove between propodeum and metapleuron absent to very weakly indicated, not u-shaped as in pionines; pleural carina present, usually strongly elevated; median longitudinal carinae very well-developed, forming flask-shaped median section with rugulose petiolar area (Fig. 5) broad and distinct, extending at least over posterior half of propodeum, petiolar area and areola sometimes delimited by transverse sculpture; lateral longitudinal carina weaker than median longitudinal carina, nearly always extending to spiracle from posterior margin but usually absent anteriorly, transverse carinae absent in three of four species examined. Legs with apical margin of mid tibia expanded into a tooth that is not quite as well-developed as that of fore leg; apical comb on posterior side of hind tibia weakly developed to absent; posterior hind tibial spur at least 0.5 times length of hind basitarsus, at least in females (Fig. 1); tarsal claws not pectinate (Fig. 8); fifth tarsomere of hing leg normal, not unusually elongate (relative to fourth) (Fig. 1). Fore wing (Fig. 6) with areolet present; stigma moderately broad, Rs+2r arising basad midpoint. Hind wing (Fig. 6) with first abscissa of CU1 nearly always longer than 1cu-a. T1 relatively short (Figs 7, 8), strongly expanding posteriorly; ventral margin straight in profile; dorsal carinae present and well-developed to about level of spiracle, absent posteriorly; basal depression at dorsal tendon attachment broad, shallow; dorsal-lateral carina complete between spiracle and apex of T1; glymma absent. S1 not extending to level of spiracle. Laterotergites of T2 and T3 separated by creases from median tergite. Ovipositor and sheath (Figs 1, 9) straight, ovipositor with distinct dorsal, subapical notch.
The above description is modified from Townes (1970), and based four females and one male in the Texas A&M University collection.